Essential medicines (2017)
Equitable and Economical Access to Effective Therapies (E3T)
Research and Markets: Availability of Essential Medicines in the Czech Republic (2017)
Availability of essential medicines in the Czech Republic
Research & Markets (2017)
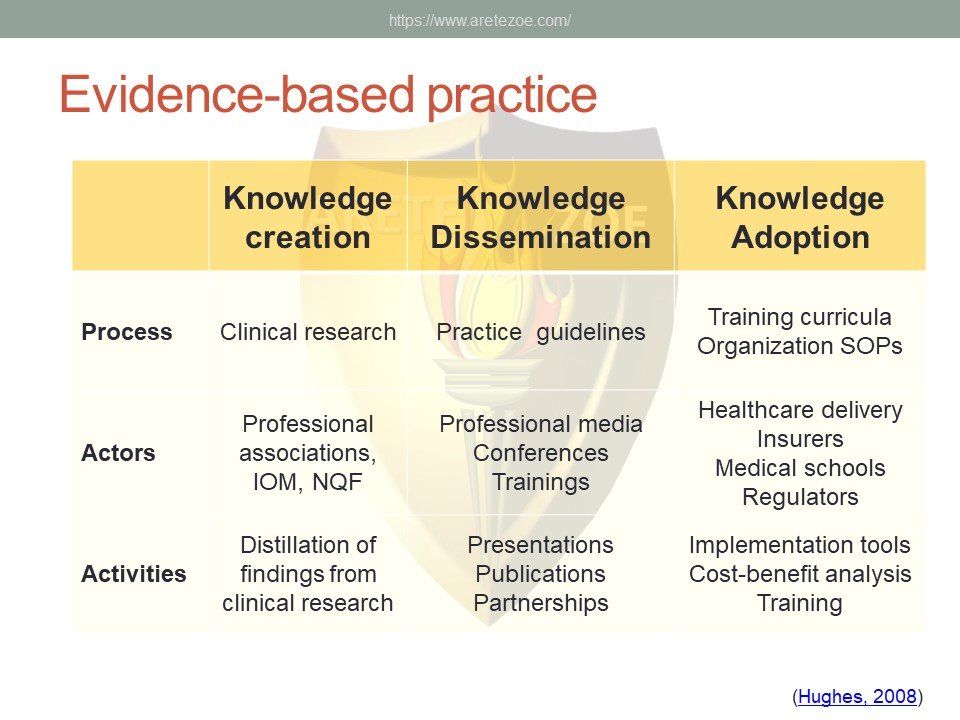
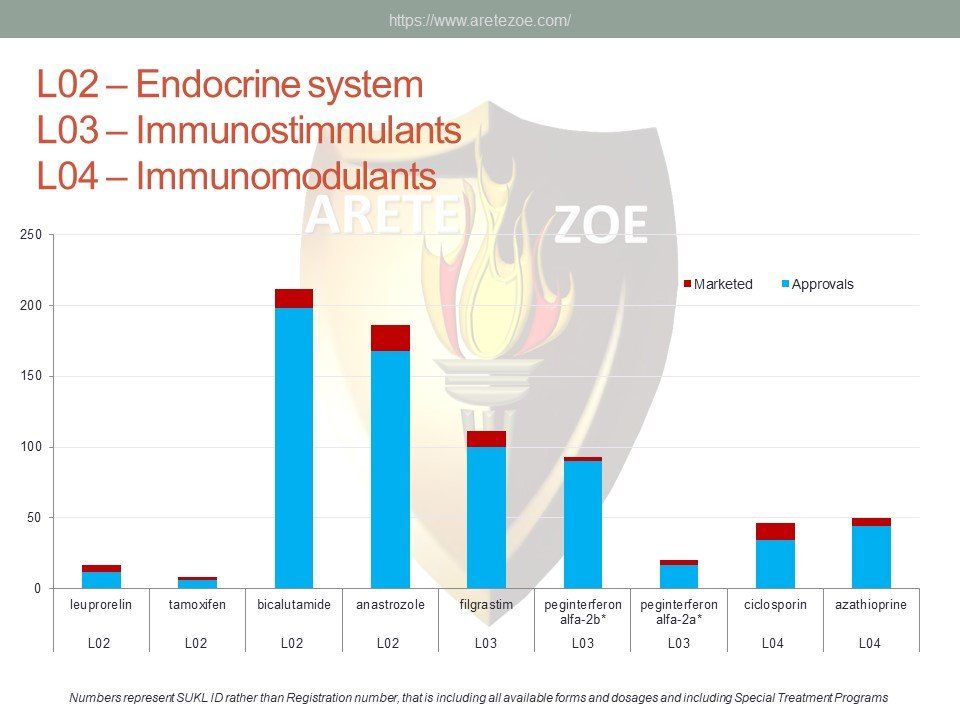
This report analyzes availability of essential medicines as defined in the World Health Organization (WHO) Essential List Medicines (The Selection And Use Of Essential Medicines. Report Of The WHO Expert Committee, 2015) in the Czech Republic. The report offers comprehensive information on active pharmaceutical ingredients offered locally as well as number of registrations for each API by system organ class. The WHO list of essential medicines contains most effective and safe medicines needed to meet the most important needs in health systems, and is frequently used by countries to create their own national lists. Without these drugs, some conditions will not be able to receive optimal therapy. Availability gap represents serious public health concern. Of the total number of 13,256 individual registrations for essential medicines, only 2,110 (14%) were actively marketed in Q3 2016.
Availability of Essential Medicines in the Czech Republic
Availability of essential medicines in the Czech Republic
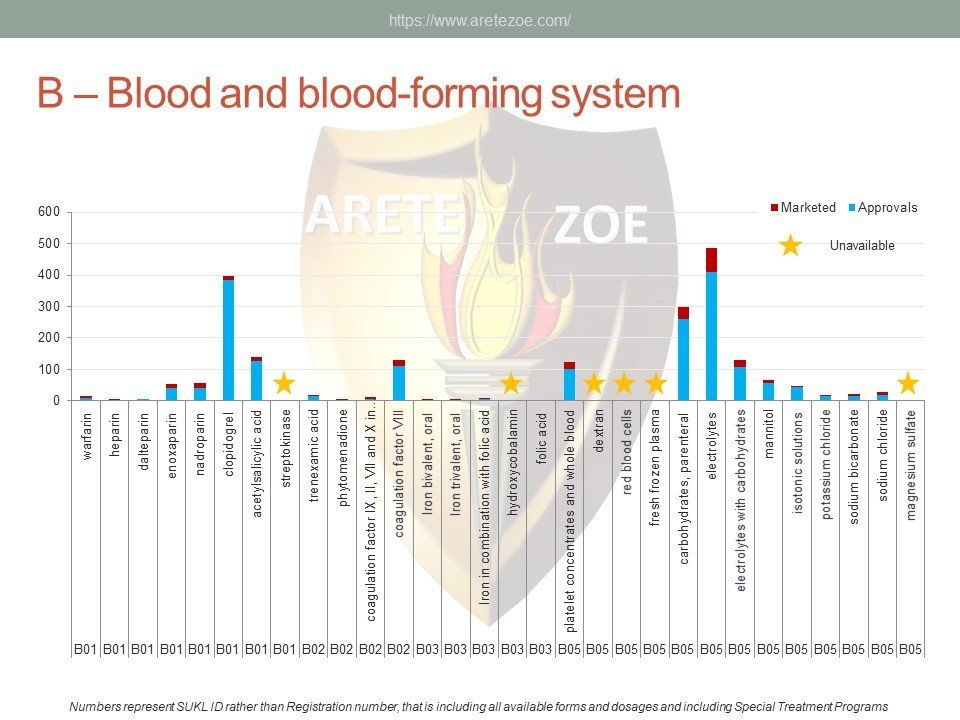
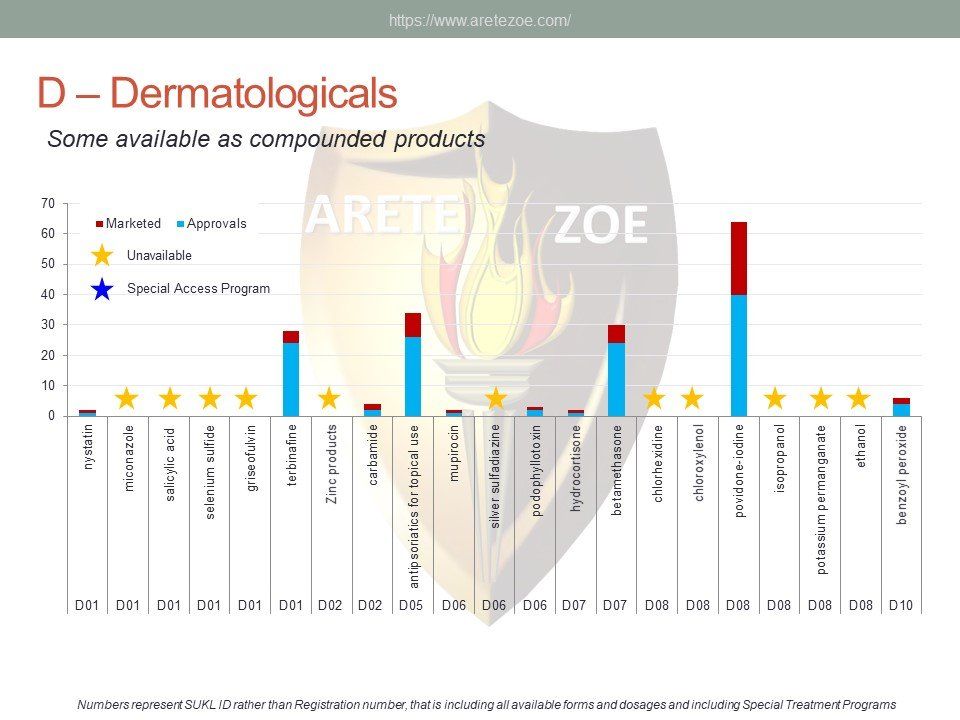
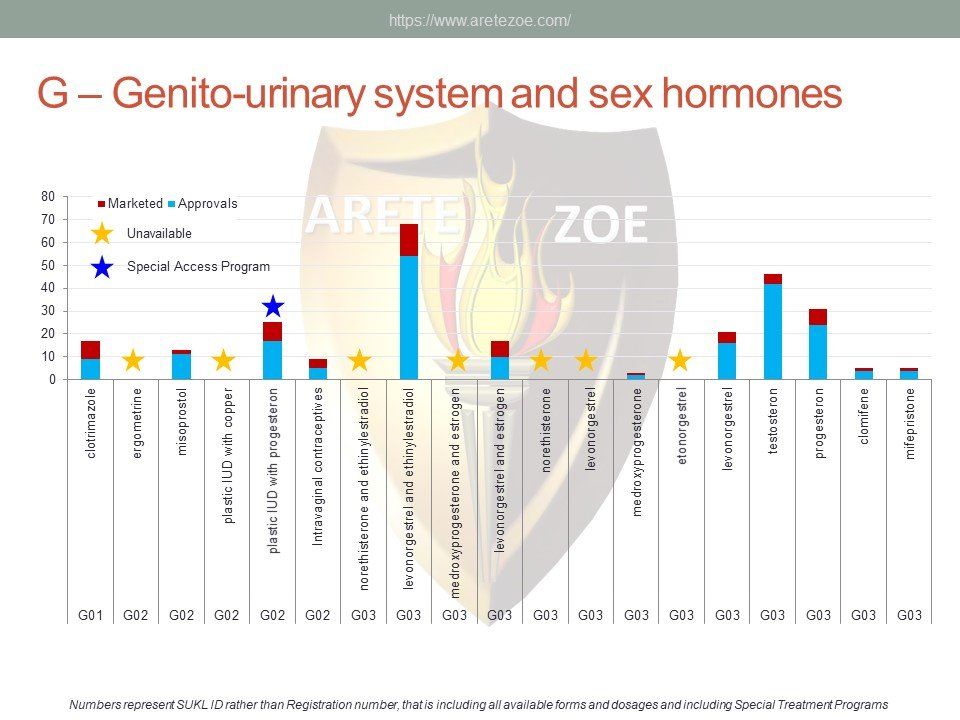
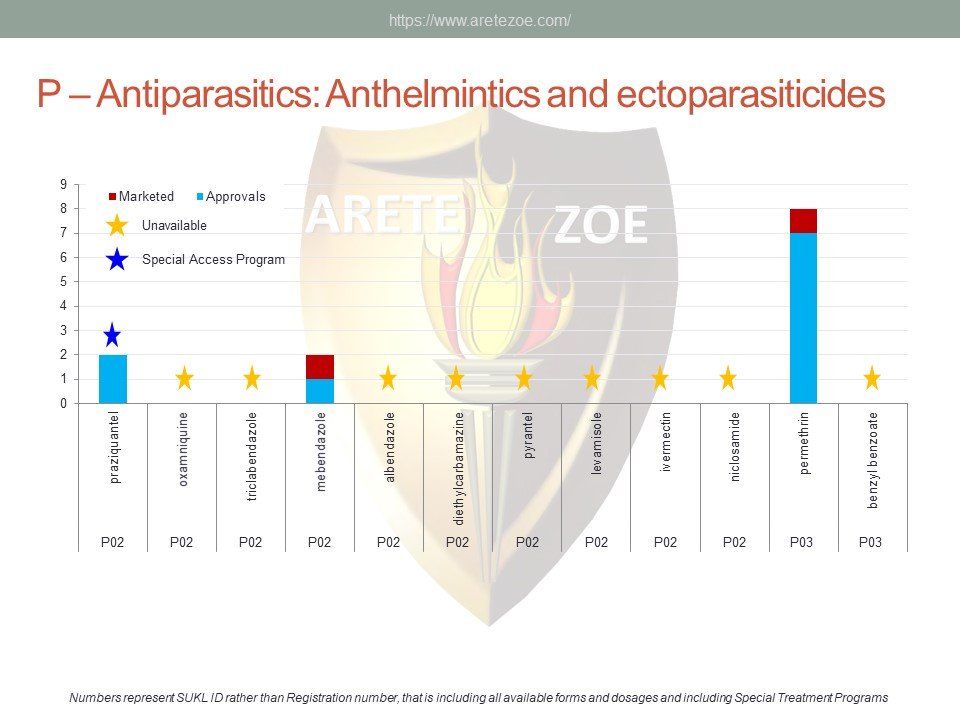
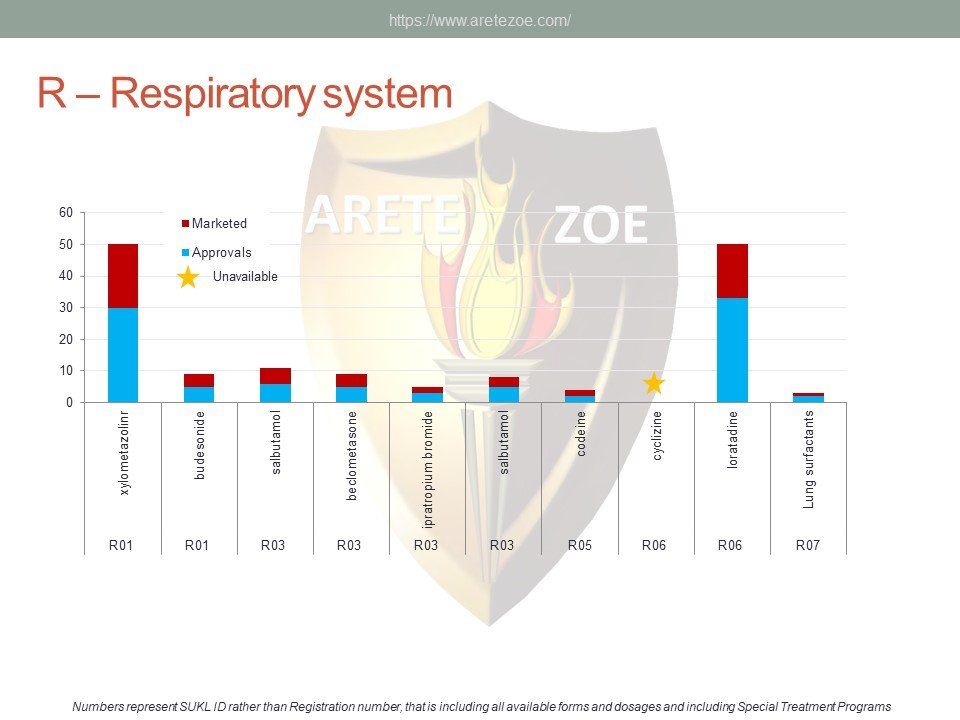
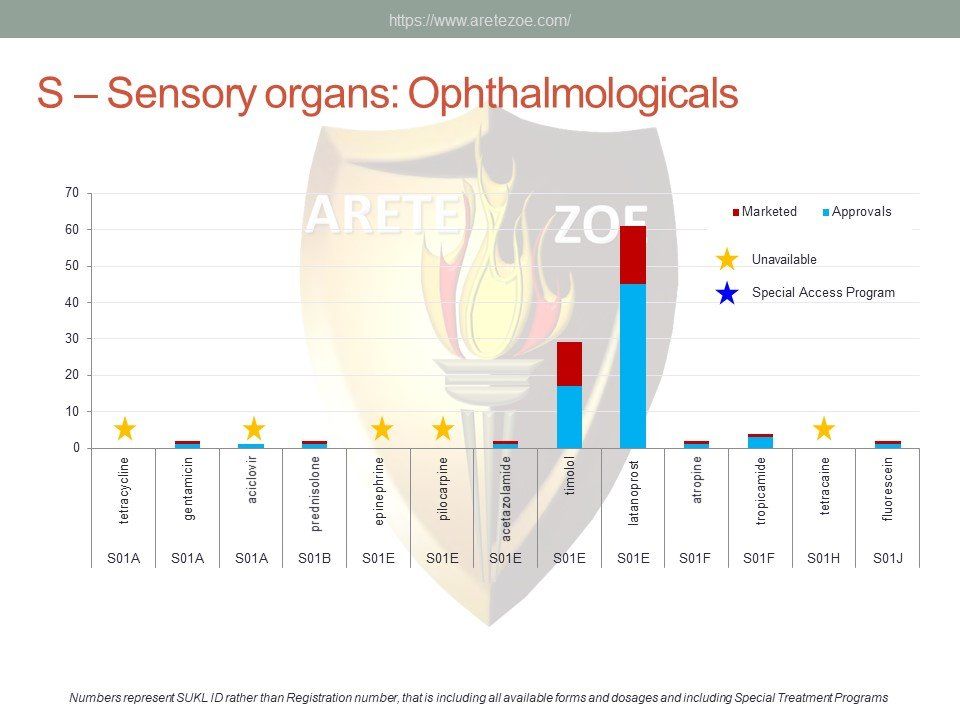
The World Health Organization (WHO) list of 427 essential medicines was compared to medicinal products available in the Czech Republic. Availability was judged by authorization status and presence on the market. The dataset was downloaded from national database of medicinal products on December 30, 2016. Of the 427 essential medicines defined by WHO, 135 are currently (as of December 30, 2016) unavailable in the Czech Republic. The most affected pharmacological groups are antiparasitics, antiinfectives, and dermatologicals.
Articles published in Remedia (2017): Availability of medicines in the Czech Republic
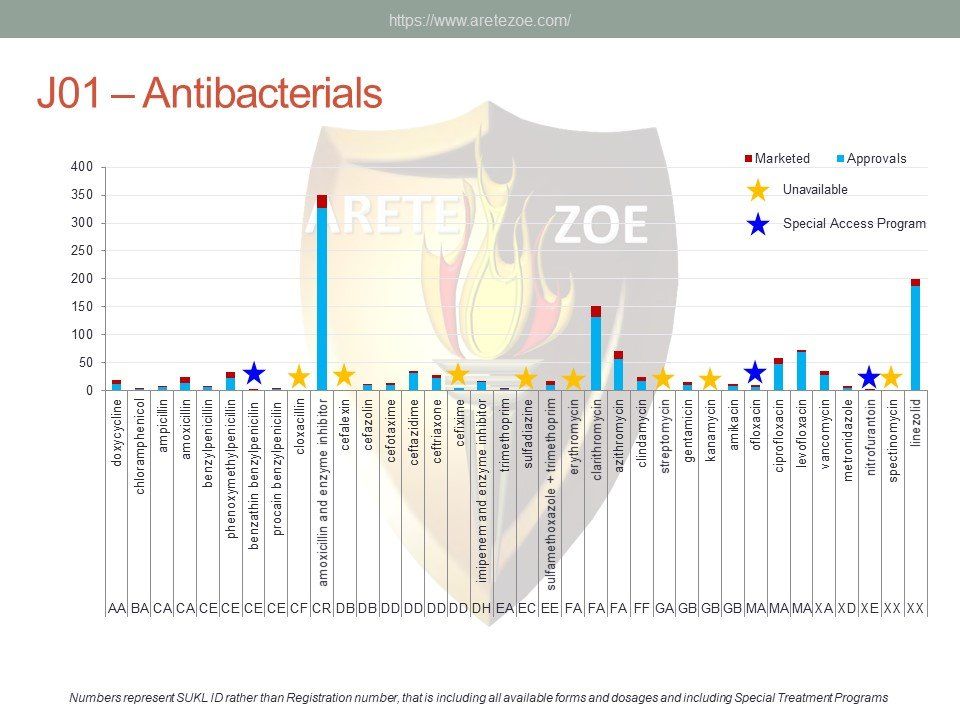
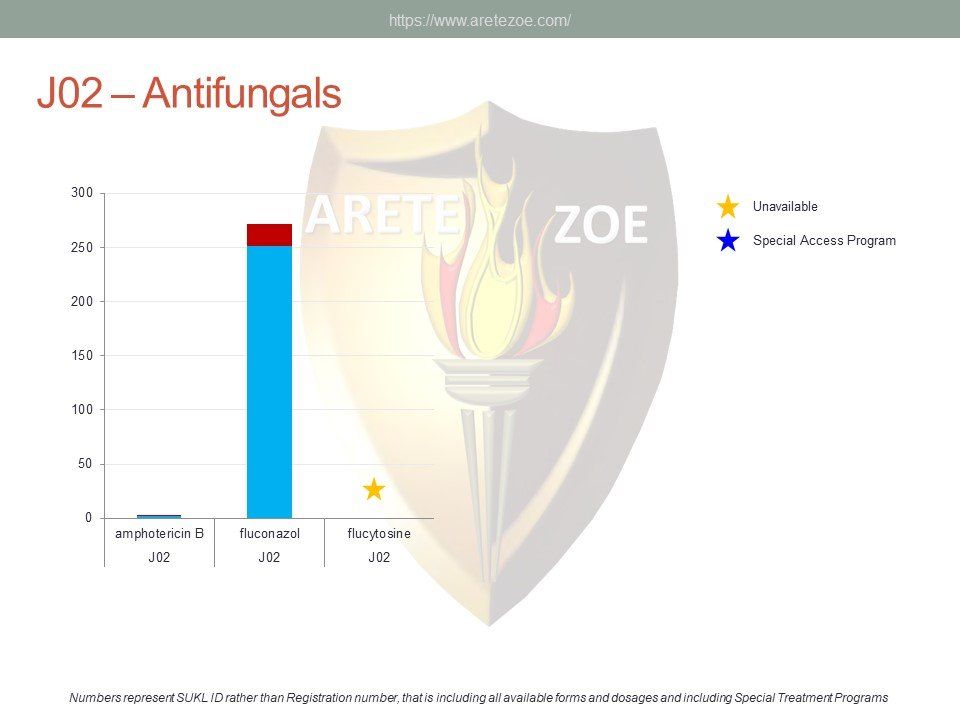
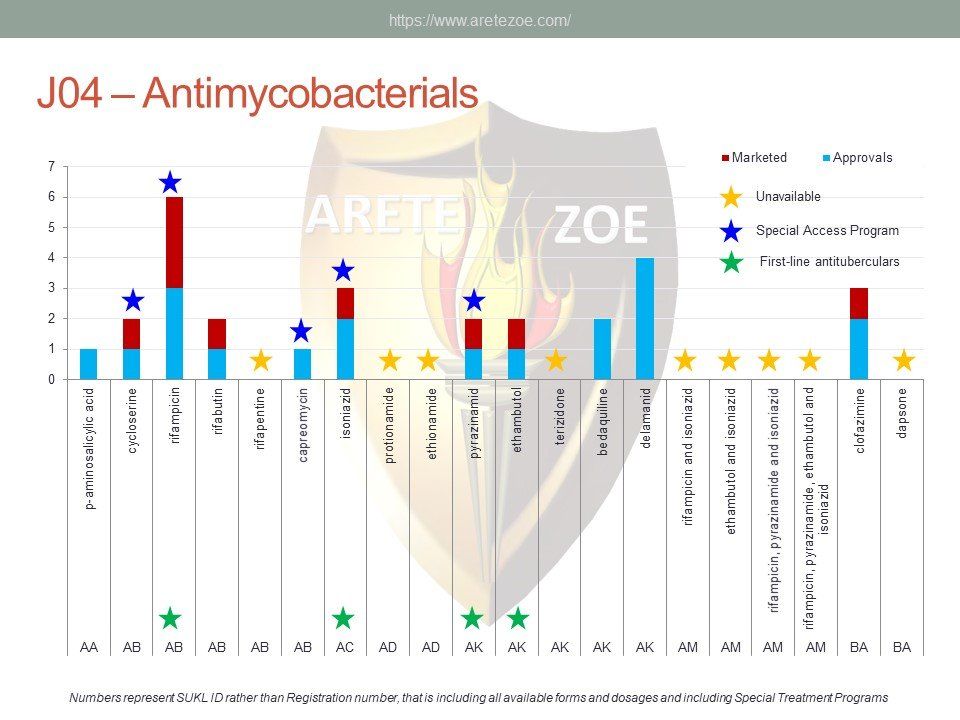
Availability of antiinfectives in the Czech Republic
Veronika Valdova (Remedia, 2017)
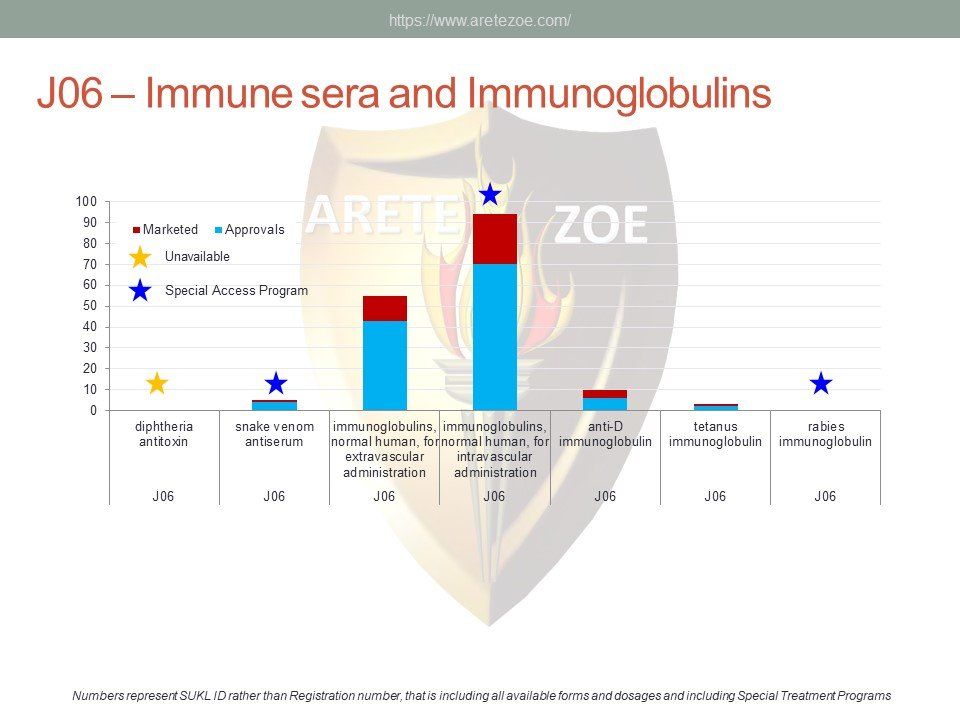
The World Health Organization (WHO) lists 119 anti-infectives on the list of essential medicines. Of these, 29 are not registered in the Czech Republic, and another 10 are registered but not marketed. availability of antimicrobials and other essential medicines directly affects clinical practice. One such example is the newly revised guidelines for the treatment of sepsis and septic shock, which recommends de-escalation of antibiotic treatment and targeting the causative pathogen immediately after its identification to minimize toxicity to the patient’s metabolism. Newly compiled national lists of essential medicines shall consider revised treatment guidelines and not just the WHO list. Some fixed combinations of anti-tuberculars and anti-retrovirals for first-line treatment of HIV/AIDS are unavailable in the Czech Republic. Counterfeit drugs and undertreated infections are the main drivers of microbial resistance. Diphtheria antitoxin and rabies immunoglobulin are the only immune sera unavailable in the Czech Republic. Shortage of vaccines occurs globally as a result of complexity and length of the manufacturing process.
How is the situation concerning drug availability in the Czech Republic?
Veronika Valdova (Remedia, 2017)
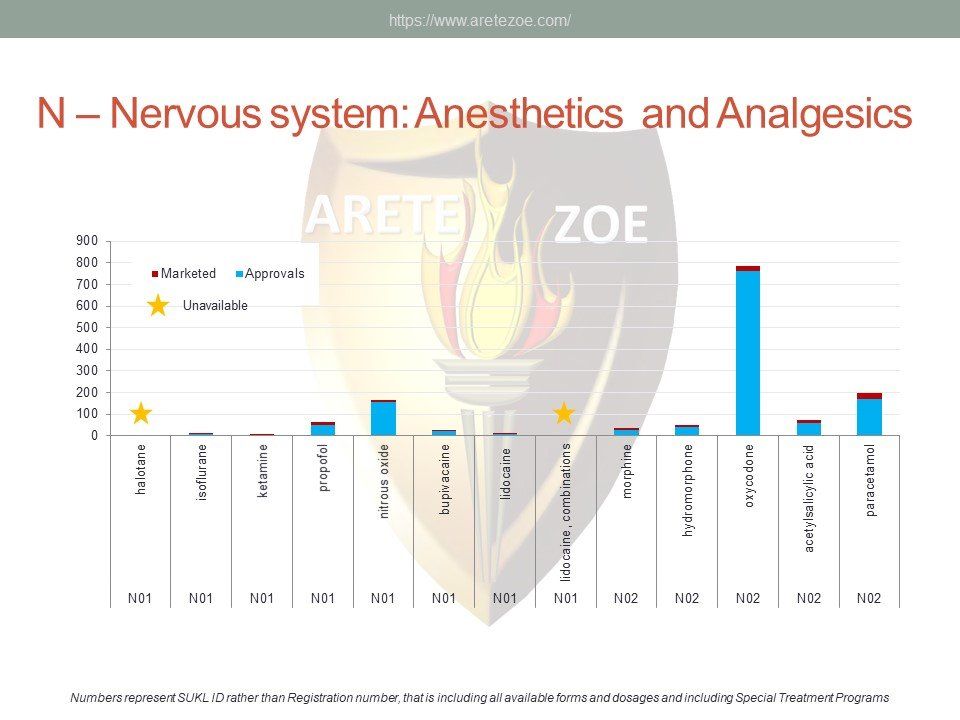
Czech Republic is a small, highly regulated EU market. Recently, concerns emerged regarding availability of certain essential medicines. Reasons for reluctance to launch products after approval include complex national regulations, lack of transparency and inconsistent enforcement, unpredictability of administrative actions, ambiguity in price negotiations, and policy shifts in response to political changes. although the current situation is mainly blamed on re‑exports, there is no comprehensive study of the extent and nature of the shortages. Data visualizations show high representation in some therapeutic areas (neurology and psychiatry, cardiovascular drugs), while other groups are hardly present at all. Shortage of certain essential medicines, especially injectables and old off‑patent medicines is a global problem. Its causes are complex and include consolidation of the industry, production issues, outsourcing of manufacturing to India and China, administrative actions against manufacturers for good manufacture practice (GMP) non‑compliance, unavailability of raw materials, trade barriers, as well as discontinuation of production for purely business reasons.
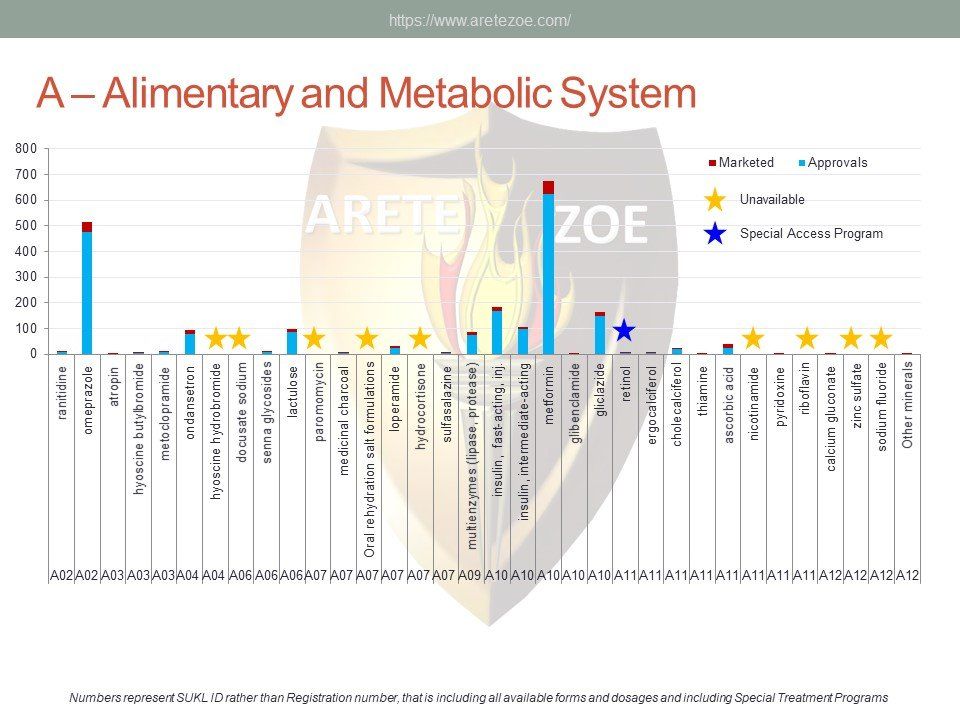
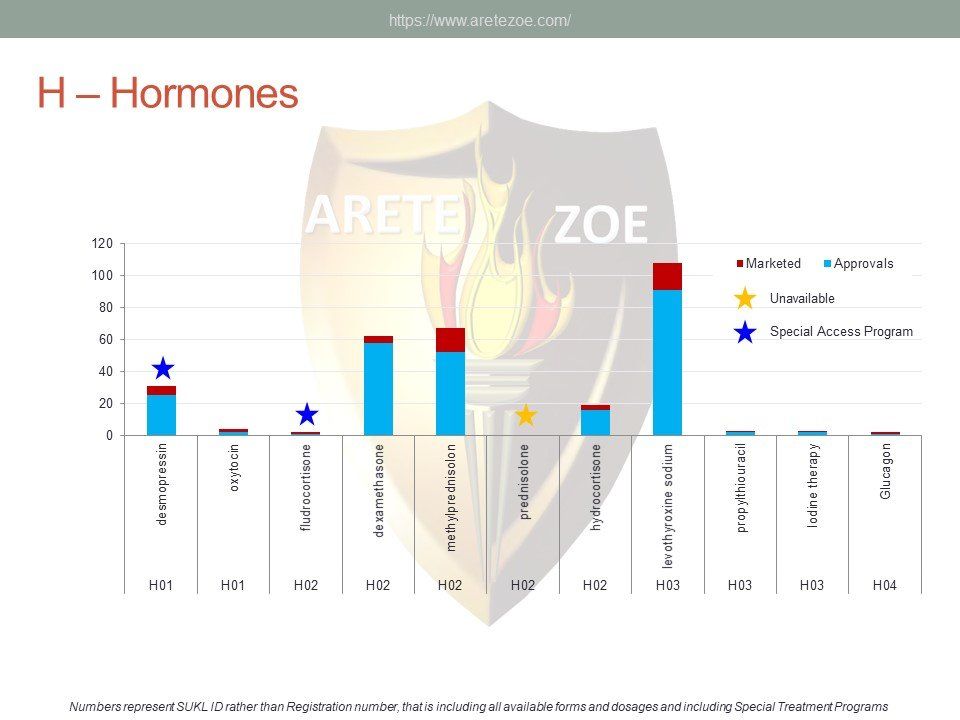
Availability of medicines used for alimentary tract and metabolic diseases treatment.
Veronika Valdova (Remedia, 2017)
The group A of the AT C classification contains products for the treatment of alimentary tract and metabolic diseases. Of the 34 drugs considered essential by the World Health Organization, 9 are not available in the Czech Republic. Some subgroups, such as drugs for treatment of functional disorders of gastrointestinal tract, are represented very sparsely. Numerous other indispensable drugs are represented only by a very limited number of registrations. Worldwide shortage of drugs pertains mainly to sterile injectable drugs, including components for parenteral nutrition. Furthermore, some important medicines listed in current guidelines, for example drugs used for treatment of H. pylori, and Cl. difficile infections, are unavailable as well. Appropriateness
of individual indication subgroups representation should be assessed by clinical specialists. Anabolic steroids and anti‑obesity medications deserve special attention due to their popularity on the black market. These drugs are easily available in the Czech Republic despite their strict regulation.
Availability of Essential Medicines in Hungary
Essential Medicines in Hungary
World Health Organization Essential Medicines List (WHO EML) are compared with the National Substitution List (NL) by system organ class. Drugs are categorized by ATC Code; that means some active ingredients are listed in all relevant forms and system organ classes. The dataset is presented as a summary and then in detail by organ class and ATC subclass as relevant/appropriate. The focus of the following analysis is on essential medicines that are not available in Hungary, and evaluation of the situation in the context of public health needs and global drug shortages. To this end, the Essential List of Medicines defined by the World Health Organization (WHO) is compared to the Substitution List, which can be found on the website of The National Institute of Pharmacy and Nutrition.
Availability of Essential Medicines in Hungary (2017)
This report analyzes availability of essential medicines as defined in the World Health Organization (WHO) Essential List Medicines (Report of the WHO Expert Committee) in Hungary. Without these essential drugs, some conditions will not be able to receive optimal therapy. Availability gap represents serious public health concern. The dataset is presented as a summary and then in detail by organ class and ATC subclass as relevant/appropriate. Focus of the following analysis is on essential medicines that are not available in Hungary, and evaluation of the situation in the context of public health needs and global drug shortages. The dataset is current as of April 6, 2017. Locally available products were compared to the WHO list of essential medicines. The material is presented in graphs and summary tabulations as listed in the table of contents. In Hungary, the most affected groups are anthelmintics, antiprotozoals, antituberculars and antibiotics.
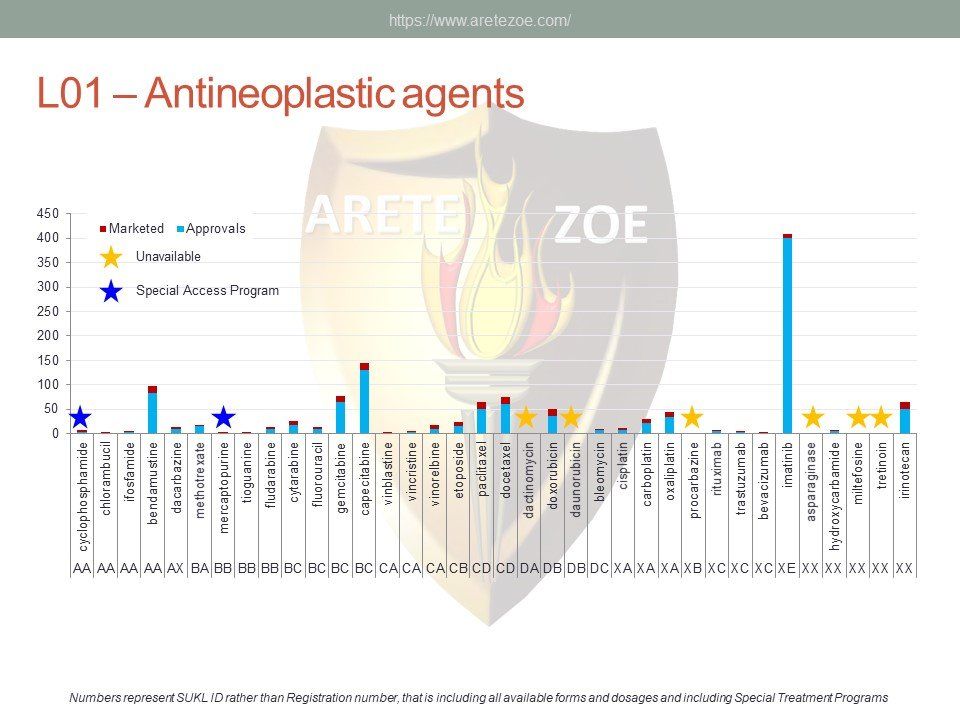
Modern Oncology Therapies in the Czech Republic
Modern oncology therapies
Availability of biological cancer therapies in the Czech Republic