One stop shop for placing your medical devices on the EU market
We assist manufacturers in preparing technical documentation for submission to EU notified bodies and national regulatory authorities to certify their medical devices in compliance with Medical Devices Regulation (EU) 2017/745. Our team has an excellent record in preparing documentation that successfully passes the certification process. Additional services include notification and follow-up with national competent authorities and notified bodies.
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
What we offer
We prepare and deliver technical documentation required for the certification of your devices on the EU market, including the Risk Management File (RMF), Clinical Evaluation (CER), Post-Market Clinical Follow-Up (PMCF), Post-Market Surveillance Plans and Reports (PMS), Periodic Safety Update Reports (PSUR), and Biocompatibility Assessment Reports. We involve a clinical expert in the relevant field as appropriate.
- Review, revision, and complete preparation of all parts of technical documentation required for successful certification of medical devices in the European Union
- Preparation of Risk Management File in compliance with ISO 14971:2019 and Medical Devices Regulation (EU) 2017/745
- Screening of scientific literature for Clinical Evaluation Reports (CERs) and Biological Compatibility Assessment Reports (BCARs)
- Preparation of Biological Compatibility Assessment Reports (BCARs) in compliance with ISO 10993-1:2018
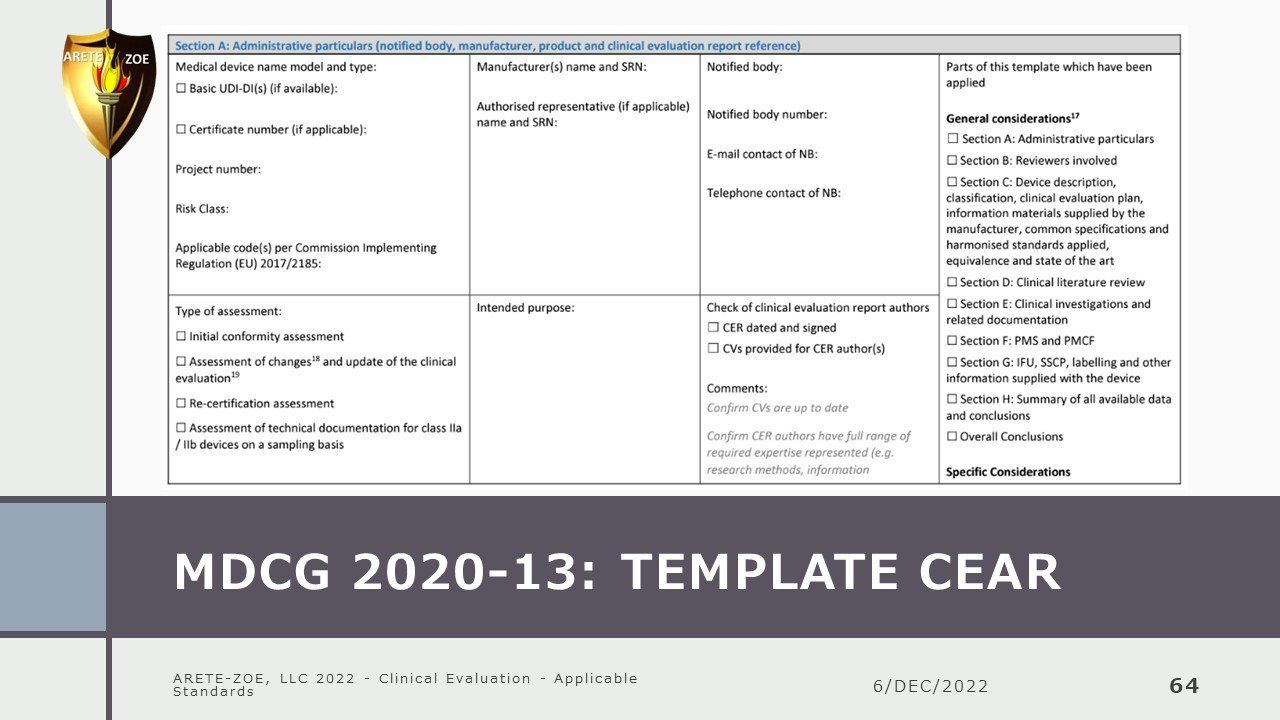
- Preparation of clinical documentation, i.e., Clinical Evaluation Plan and Report
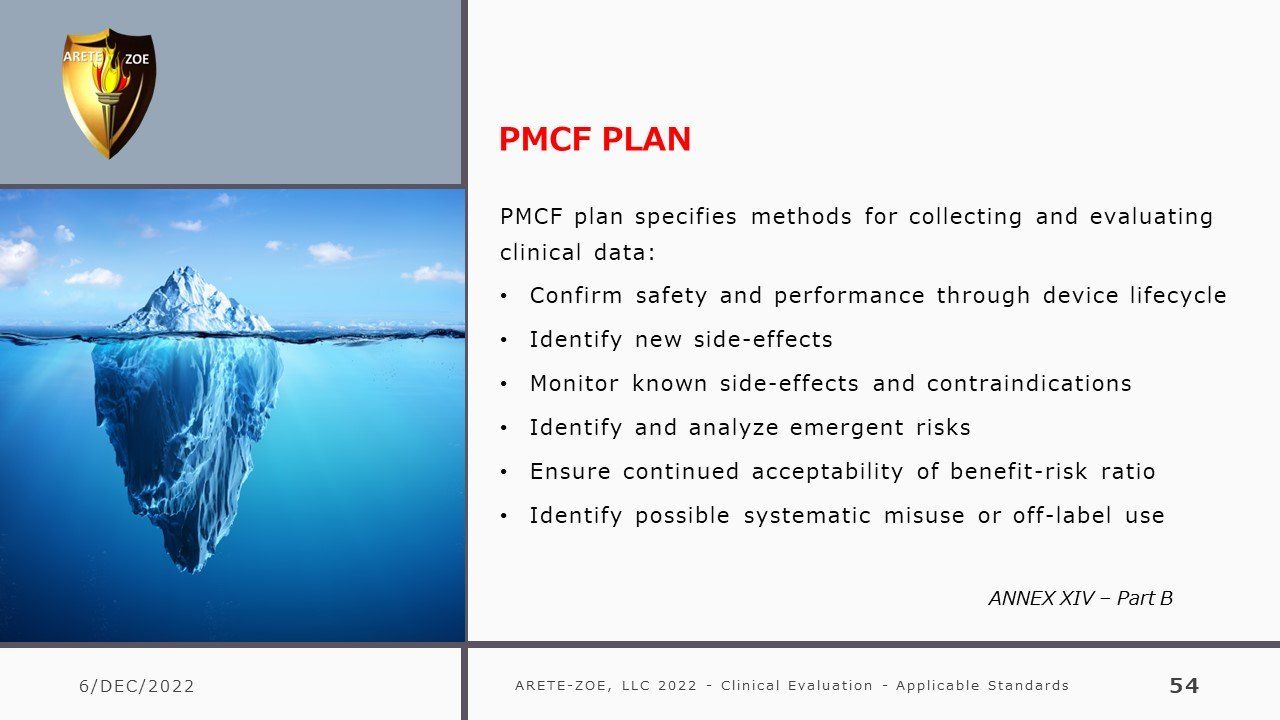
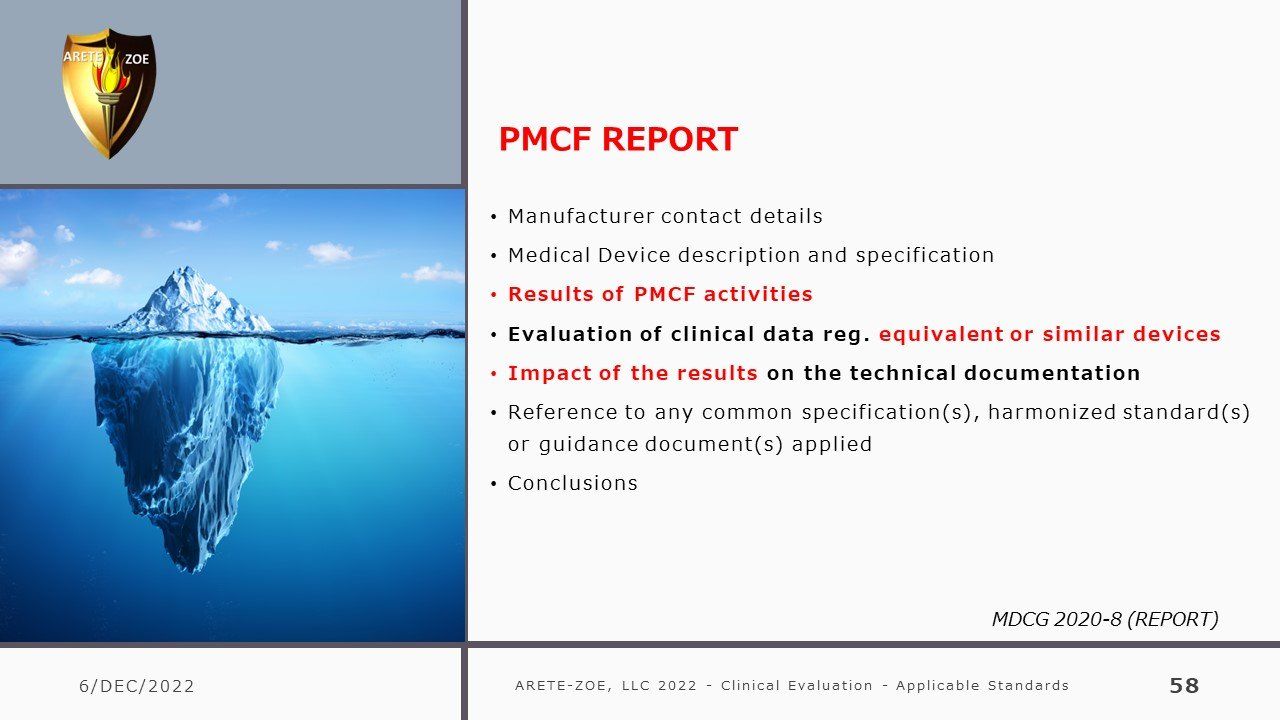
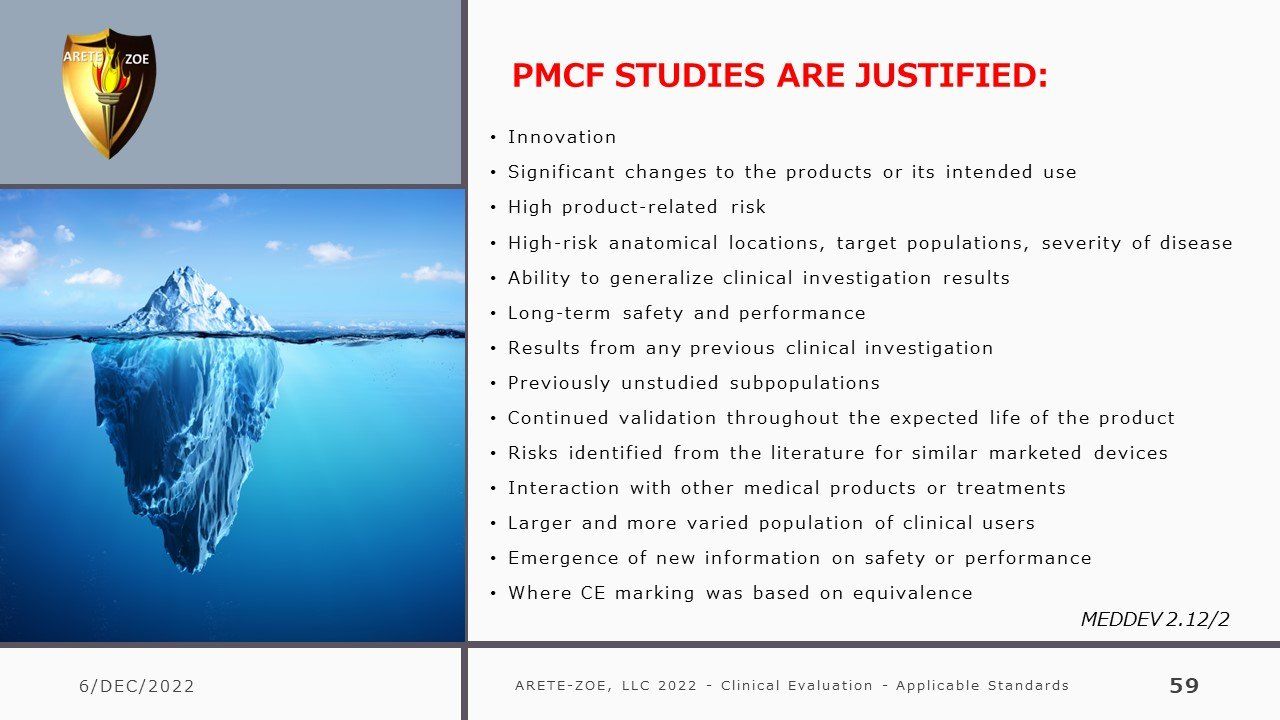
- Design of PMCF studies, preparation of Post-Market Clinical Follow-Up (PMCF) Plan and Report
- Post-Market Surveillance Plan and Report
- Periodic Safety Update Reports
- Suggestions for revision of Instructions for Use and other informational materials
- Preparation of internal training materials and processes to facilitate the transition to in house follow-up and maintenance
Our Team has an exceptional record of successful submissions.
- Strategy closely coordinated with manufacturers
- Experienced team of highly trained professionals
- Access to multiple medical specialties
- Full-spectrum of required documentation
- Attentive and professional staff with a can-do attitude
- Extensive knowledge of the regulatory environment
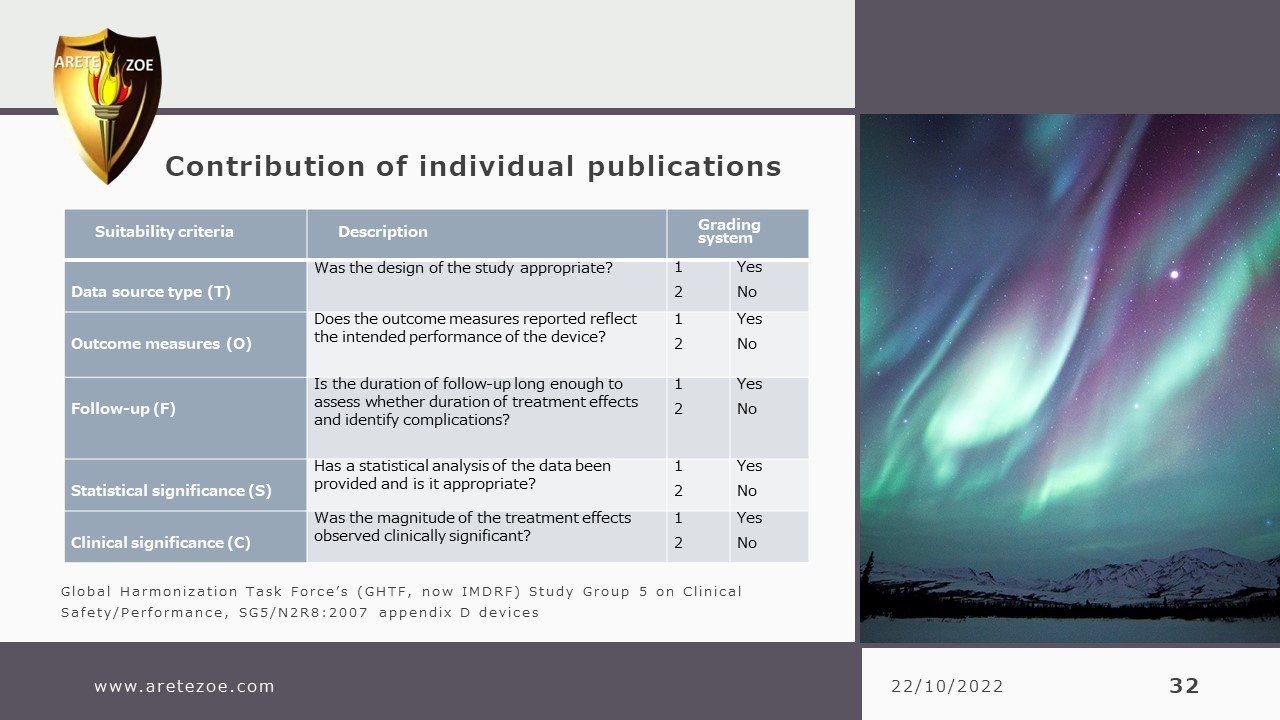
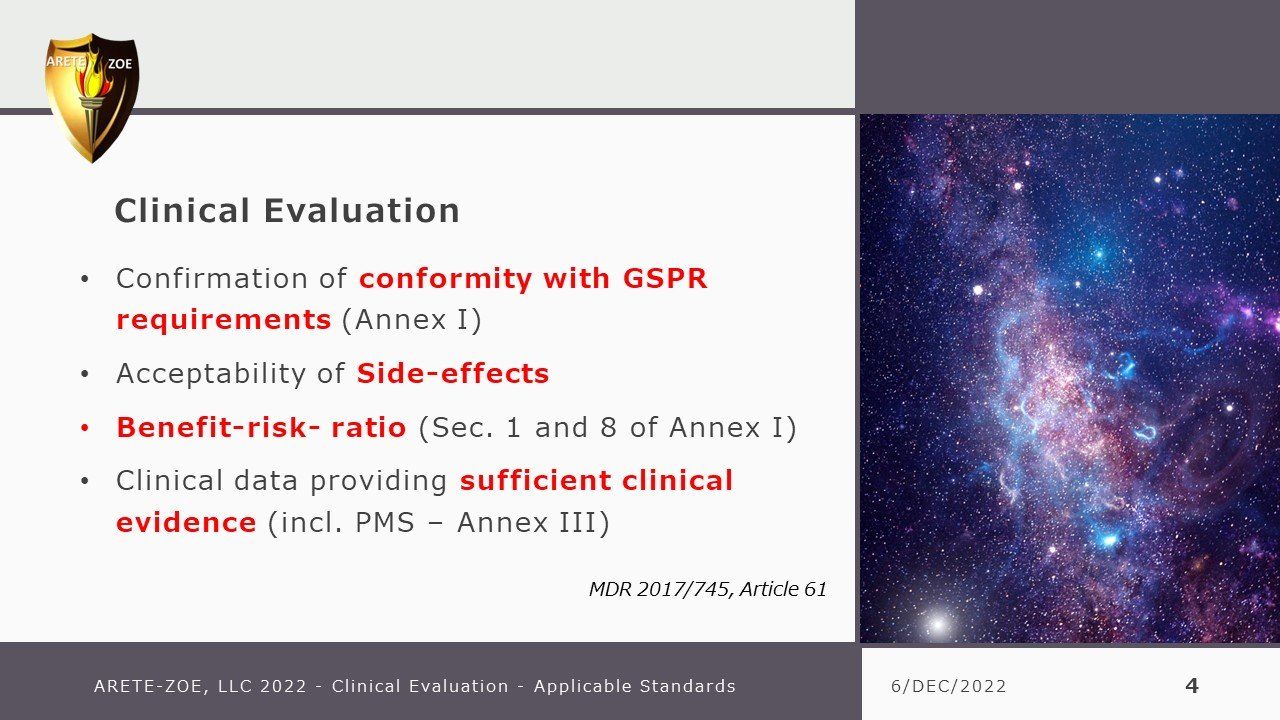
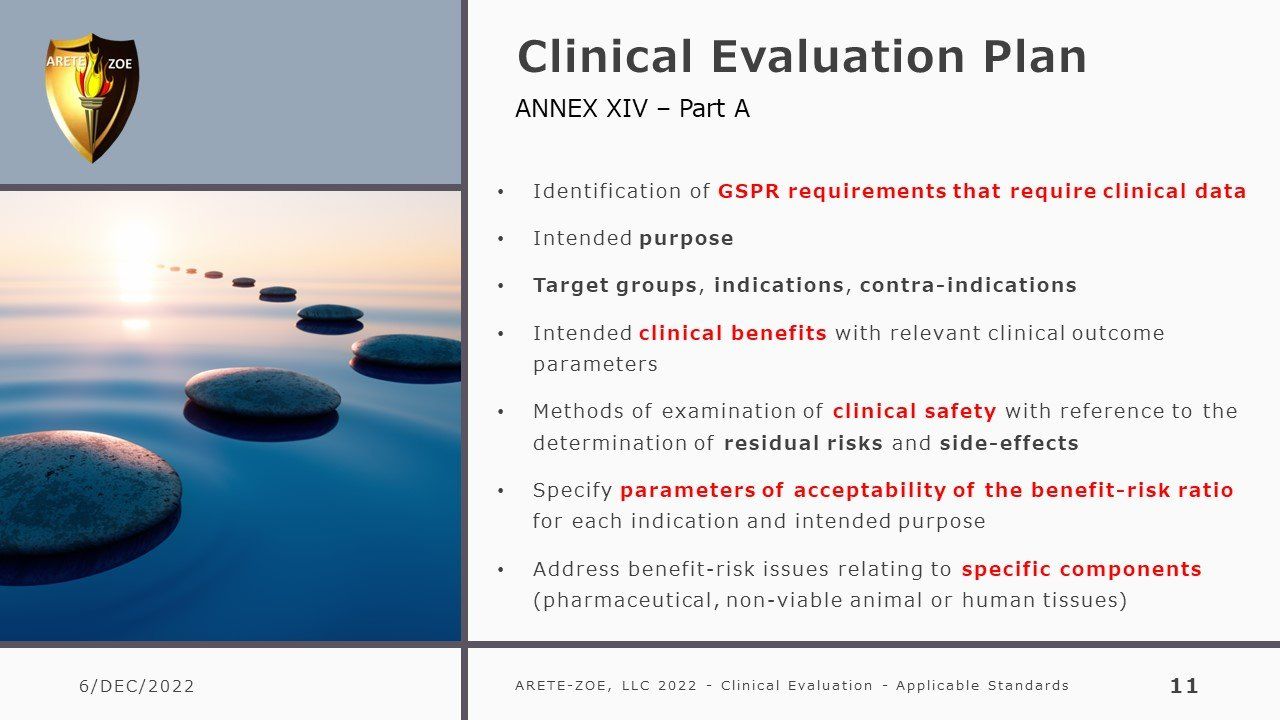
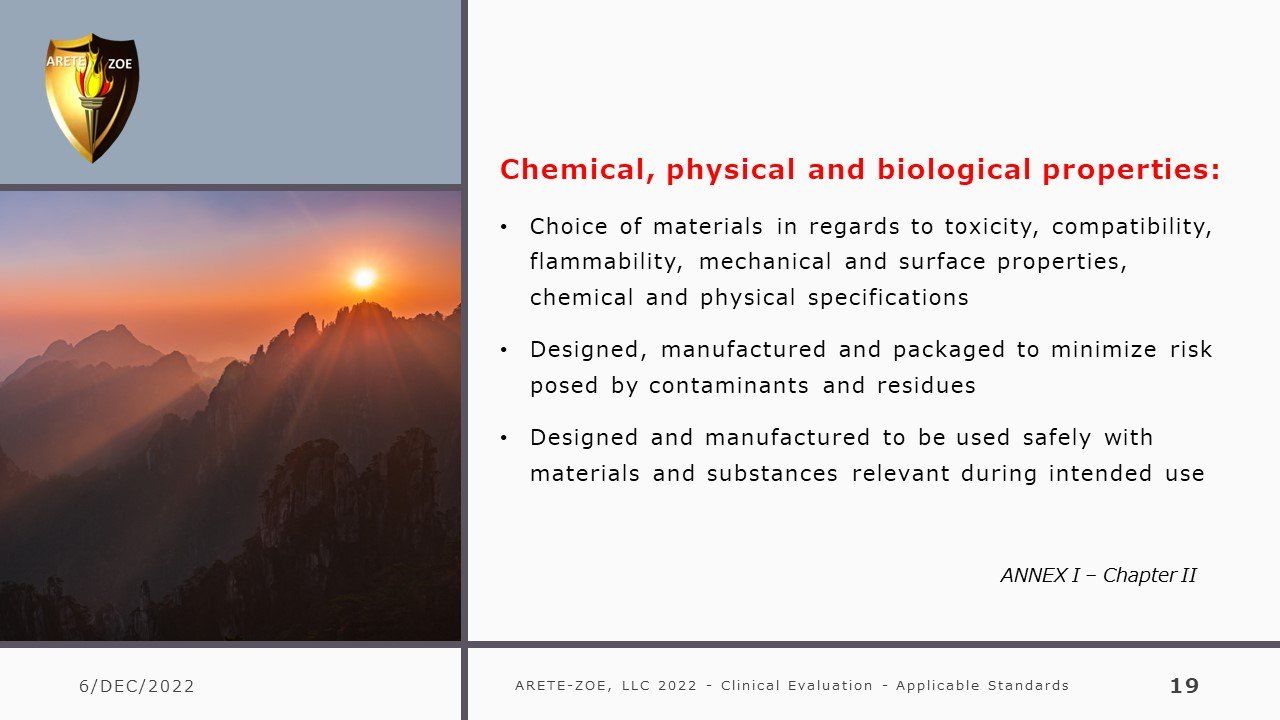
Clinical evaluation: applicable standards
Although this is a comprehensive inventory of relevant standards determining EU medical device conformity compliance for marketing certification, it is not an instruction on how to compile a successful MDR submission. There are many nuances differentiating by device class or Notified Body that individualizes each specific submission and requires optimization for confident success, avoiding delays for elaboration and rework from the Notified Body. It is not as simple as filling out a new template and requires a discerning review of both past documentation and ongoing information collecting integral to the manufacturing process including compare and contrast of safety and efficacy of like items.
Slide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButtonSlide title
Write your caption hereButton
Contact Us
We will get back to you as soon as possible.
Please try again later.